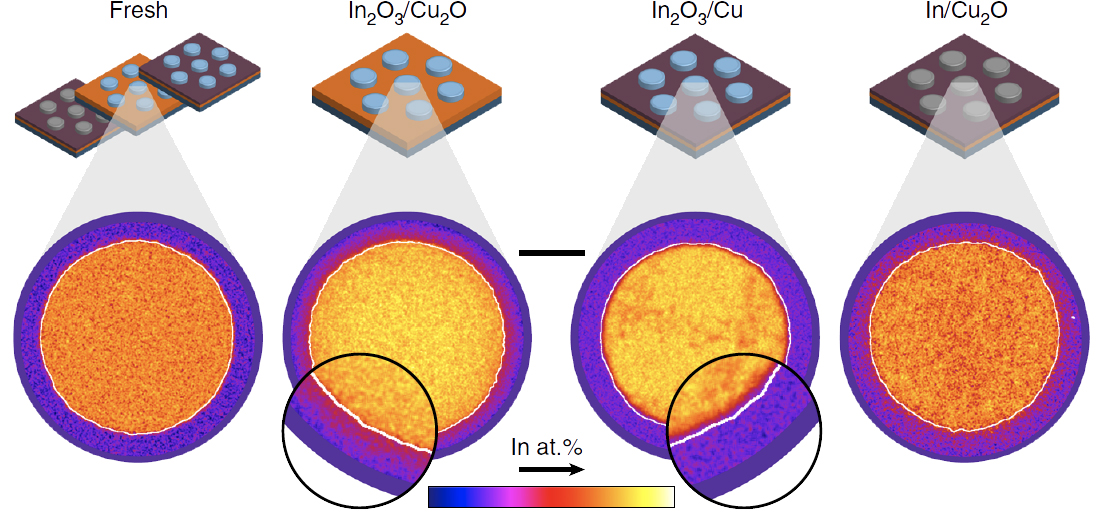
 Our A-LEAF partners at ETH Zürich, lead by Prof. Javier Pérez-Ramírez, have discovered the key participation of the interface in the catalytic activity of In/Cu electrodes for the selective and efficienct CO2 reduction reaction toward CO. This result was possible thanks to a novel experimental approach developed by Prof. Pérez-Ramírez’s team. This approach consists in the deposition of microscale structures (In or In2O3, in this case), patterned by ultraviolet (UV) photolithography, on the desired support (Cu or Cu2O, in this case). The relation between the catalytic activity the geometry and composition of the electrodes, in combination with microscopy, allowed linking the electrocatalytic activity to the density of indium-poor bimetallic phases formed at the vicinity of the indium–copper interfaces upon exposure to reaction conditions.
Our A-LEAF partners at ETH Zürich, lead by Prof. Javier Pérez-Ramírez, have discovered the key participation of the interface in the catalytic activity of In/Cu electrodes for the selective and efficienct CO2 reduction reaction toward CO. This result was possible thanks to a novel experimental approach developed by Prof. Pérez-Ramírez’s team. This approach consists in the deposition of microscale structures (In or In2O3, in this case), patterned by ultraviolet (UV) photolithography, on the desired support (Cu or Cu2O, in this case). The relation between the catalytic activity the geometry and composition of the electrodes, in combination with microscopy, allowed linking the electrocatalytic activity to the density of indium-poor bimetallic phases formed at the vicinity of the indium–copper interfaces upon exposure to reaction conditions.
This work, “Microfabricated electrodes unravel the role of interfaces in multicomponent copper-based CO2 reduction catalysts” was published (Open Access) on April 16th in Nature Communications, 9, 1477, doi:10.1038/s41467-018-03980-9, and it is also available in our A-LEAF repository. This represents the first A-LEAF contribution to the the prestigious Nature family of journals from Springer-Nature, and a significant advance in the understanding of this complex catalytic reaction, key step for the realization of artificial photosynthesis. 

